Abstract
Introduction: In the setting of allogeneic hematopoietic cell transplant (alloHCT), reported outcomes in obese patients have been conflicting (Willis H., BBMT 2010; Jian, JCO 2020). Among factors that may impact outcomes in obese patients is the dosing of conditioning chemotherapy, which is traditionally performed using body mass or body surface area. Some dosing protocols utilize adjusted or ideal body weight rather than actual body weight, thus resulting in a lower relative dose of chemotherapy in obese patients. Data evaluating the impact of these adjustments is limited in obese patients. The aims of this study are to (1) compare the outcomes of obese and non-obese patients with acute myeloid leukemia (AML) who underwent alloHCT, and (2) evaluate the impact of dose adjustments for melphalan when used in conditioning chemotherapy.
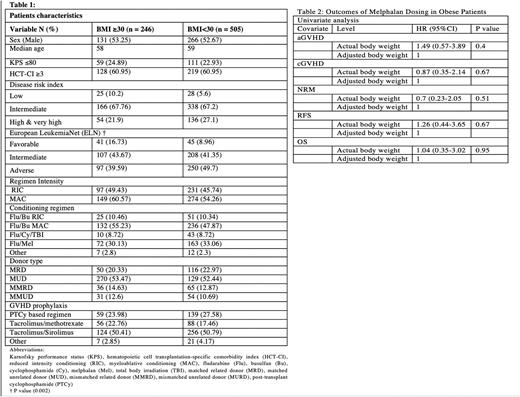
Methods: We retrospectively evaluated 751 consecutive patients with AML who underwent alloHCT at the Moffitt Cancer Center from January 2010 to December 2021. The objectives were to compare engraftment, acute GVHD (aGVHD), chronic GVHD (cGVHD), relapse, non-relapse mortality (NRM), relapse free survival (RFS) and overall survival (OS), based on body mass index (BMI) kg/m2 (< 30 vs. ≥ 30). In the subgroup of patients with BMI ≥ 30 who received fludarabine/melphalan (Flu/Mel), we compared outcomes based on melphalan dosed using actual vs. adjusted body weight.
Results: Baseline characteristics of 751 patients included in this study are shown in Table 1. Median age at transplant was 58 years (range, 18-78). Median BMI was 27.5 kg/m2 (range, 15-55.2) with BMI of ≥ 30 kg/m2 in 246 (32.8%) and < 30 kg/m2 in 505 (67.2%) patients. Reduced intensity regimens (RIC) were given to 97 (39.4%) patients with BMI ≥ 30 and 231 (45.7%) patients with BMI < 30.
For the entire cohort, neutrophil engraftment was achieved in 98.7% and platelet engraftment was achieved in 89.9%. The cumulative incidence of grade 2-4 aGVHD at 100 days was 45% and of moderate to severe cGVHD at 2 years was 36%. The relapse rate at 2 years was 26% (95% CI 23 - 29%) and 1-year NRM was 14%, (95% CI 12 - 17%). The 2 years RFS was 56% (95% CI 52 - 60%) with a median RFS of 49.2 months and the 2 the years OS was 61% (95% CI 57 - 64%) with a median OS of 52.4 months.
In Multivariable analysis, BMI did not significantly influence aGVHD, cGVHD, relapse, NRM, RFS, or OS. Patients who received tacrolimus/sirolimus for GVHD prophylaxis had higher risks of aGVHD [HR=1.66, 95% CI (1.21 - 2.28), p=0.021] and cGVHD [HR=1.59, 95% CI (1.05 - 2.39), p=0.028], while patients who received tacrolimus/methotrexate had higher risk of aGVHD [HR=1.54, 95% CI (1.06 - 2.22), p=0.002] when compared with post-transplant cyclophosphamide based regimens. Compared with European LeukemiaNet (ELN) favorable risk group, adverse risk group was associated with higher relapse [HR=2.59, 95% CI (1.47 - 4.57), p=<0.001], worse RFS [HR=2.05, 95% CI (1.33 - 3.16), p=0.001] and worse OS [HR=2.34, 95% CI (1.47 - 3.73), p<0.001]; whereas intermediate risk group was only associated with worse OS [HR=1.61, 95% CI (1 - 2.57), p=0.048]. Myeloablative fludarabine/busulfan (Flu/Bu) was associated with higher rates of cGVHD [HR=1.51, 95% CI (1.14 - 1.99), p=0.004], relapse [HR=2.59, 95% CI (1.77 - 3.79), p<0.001] and NRM [HR=0.44, 95% CI (0.31 - 0.64), p<0.001] when compared with Flu/Mel. Reduced intensity Flu/Bu was associated with higher relapse [HR=3.08, 95% CI (1.89 - 4.99), p=<0.001], worse RFS [HR=1.76, 95% CI (1.23 - 2.51), p=0.002] and OS [HR=1.67, 95% CI (1.16 - 2.4), p=0.006] when compared with Flu/Mel. Patients with KPS > 80 were at lower risk of NRM [HR=0.68, 95% CI (0.48 - 0.95), p=0.026] compared with those with KPS ≤80.
In subgroup analysis of patients BMI ≥ 30 who received Flu/Mel, there were no differences in aGVHD, cGVHD, relapse, NRM, RFS or OS when dosing melphalan based on actual body weight (n=64) vs adjusted body weight (n=14). Table 2.
Conclusion: In our cohort of patients with AML receiving alloHCT, BMI ≥ 30 was not associated with inferior outcomes compared to BMI < 30. In the subgroup of patients with BMI ≥ 30 receiving Flu/Mel, outcomes were similar between melphalan dosed using adjusted body weight and actual body weight.
Disclosures
Faramand:Kite/Gilead: Research Funding; Novartis: Research Funding. Lazaryan:Teladoc: Current equity holder in publicly-traded company; AvroBio: Consultancy; Sanofi: Consultancy; Humanigen: Consultancy; AmWel: Current equity holder in publicly-traded company. Hansen:Survivorship: Honoraria; BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; OncLive: Honoraria. Liu:Sanofi: Speakers Bureau. Pidala:Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Takeda: Research Funding; Johnson and Johnson: Research Funding; Pharmacyclcis: Research Funding; Abbvie: Research Funding; BMS: Research Funding. Locke:ASH: Other: Education or editorial activity; Takeda: Consultancy; Imedex: Other: Education or editorial activity; Society for Immunotherapy of Cancer: Other: Education or editorial activity; Clinical Care Options Oncology: Other: Education or editorial activity; Sana: Consultancy; BMS: Research Funding; Daiichi Sankyo: Consultancy; Aptitude Health: Other: Education or editorial activity; Leukemia and Lymphoma Society: Research Funding; ), National Cancer Institute: Research Funding; CERo Therapeutics: Research Funding; CAREducation: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Bejanyan:Medexus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; CareDX Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees. Elmariah:Bristol Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal